C-CO-CO2-Na2CO3-NaCl-AgClпомогите , объясните как делать :(завтра контроха (((( - Школьные Знания.com
40. A 2g sample containing Na2CO3 and NaHCO3 loses 0.248g when heated to 300^° c,the temperature at which NaHCO3 decomposes to Na2CO3,CO2 AND H2O.what is the precentage of Na2CO3 in the given

6.2g of a sample containing Na2CO3, NaHCO3 and non-volatile inert impurity on gentle heating loses 5% of its mass due to reaction 2NaHCO3 rarr Na2 CO3 + H2 O + CO2 .

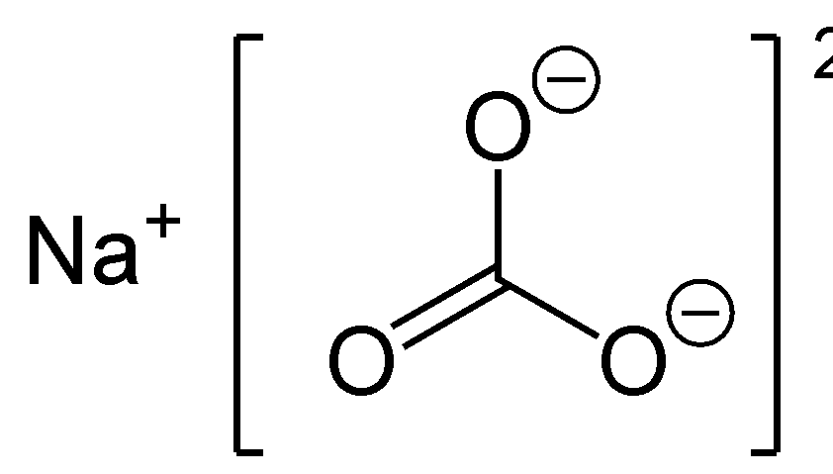
![ANSWERED] Which equation represents a non-oxidatio... - Inorganic Chemistry ANSWERED] Which equation represents a non-oxidatio... - Inorganic Chemistry](https://media.kunduz.com/media/sug-question/raw/62112503-1657345217.6842966.jpeg)