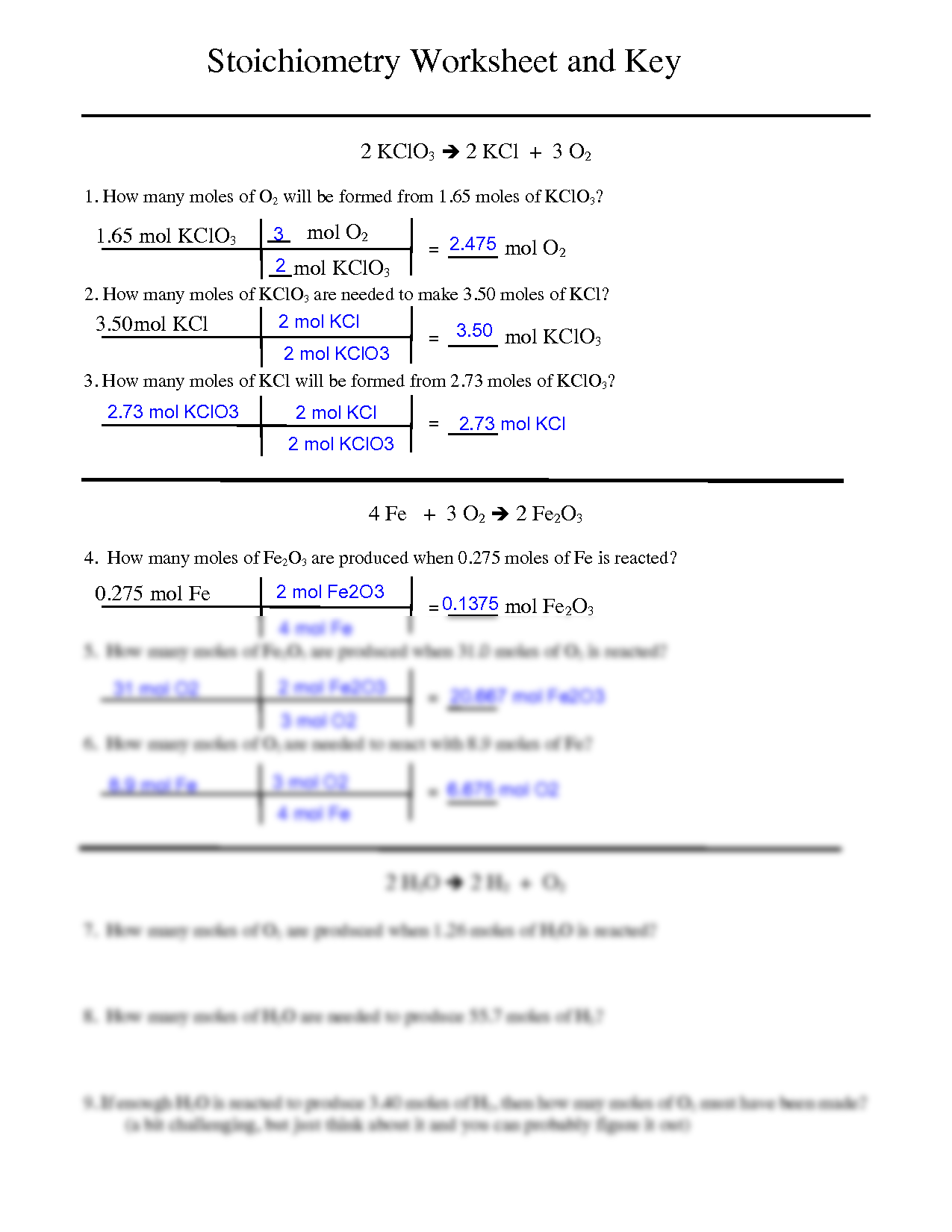
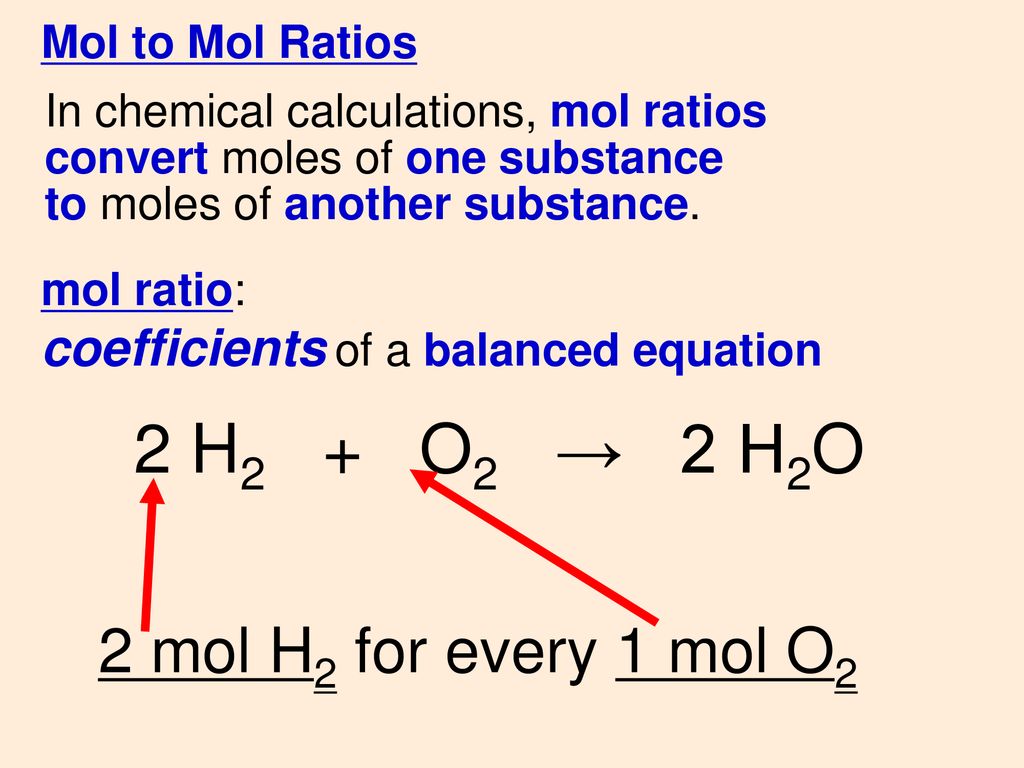
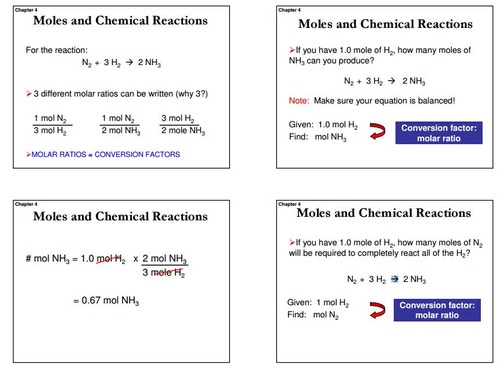
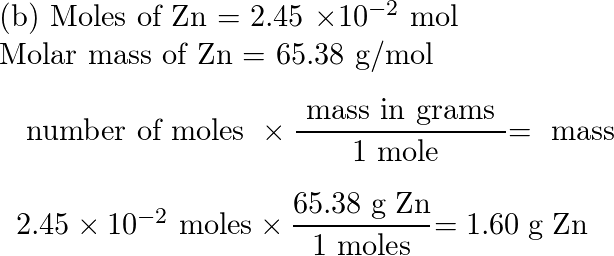Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download

For one mole of a Van der Waals gas when b = 0 and T = 300 K, the PV vs 1V plot is shown above. The value of the Van der
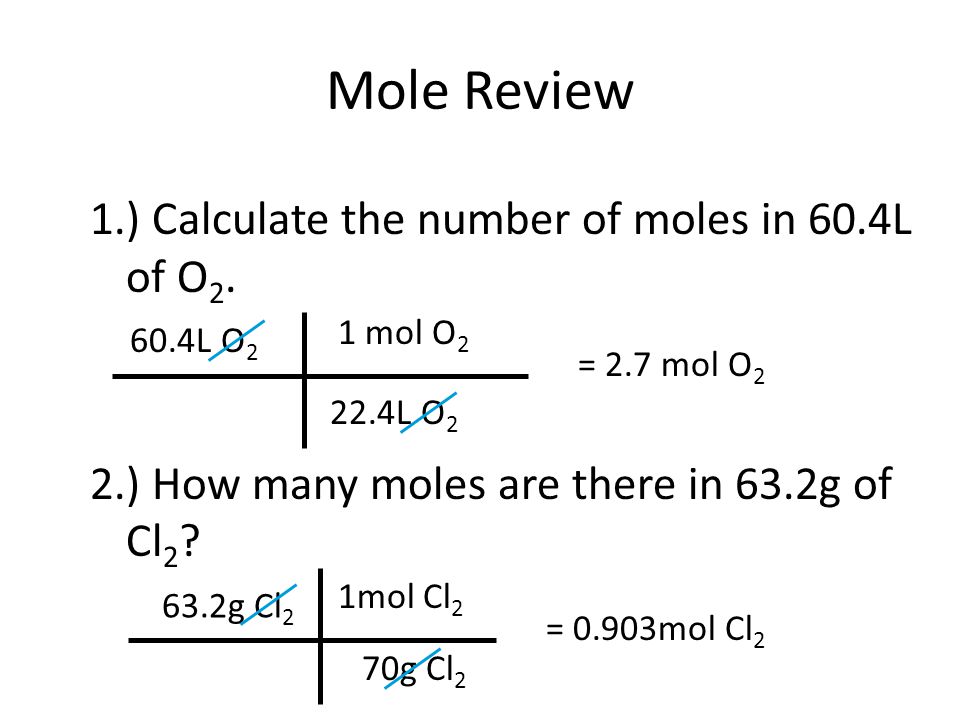
Mole Review 1.) Calculate the number of moles in 60.4L of O2. 2.) How many moles are there in 63.2g of Cl2? 1 mol O2 60.4L O2 = 2.7 mol O2 22.4L

SOLVED: What is the formula weight (in g/mol) of copper(II) nitrate trihydrate, Cu(NO3)2•3H2O? Give your answer to 6 significant figures. g/mol What is the percentage of copper (by mass) in copper(II) nitrate

Question Video: Identifying a Precipitating Agent for the Gravimetric Analysis of Chloride Ions | Nagwa