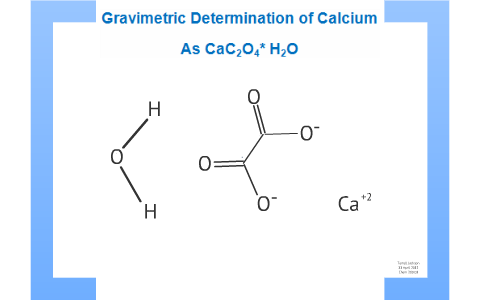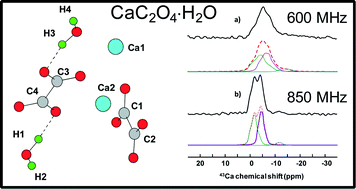
Whewellite, CaC2O4⋅H2O: structural study by a combined NMR, crystallography and modelling approach - CrystEngComm (RSC Publishing)
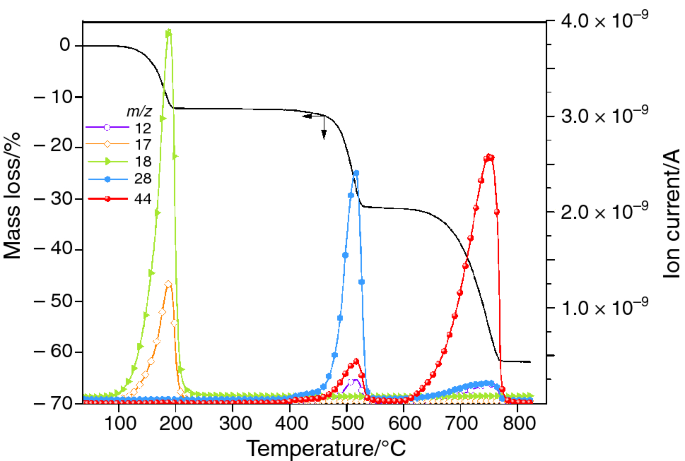
Thermal Stability of Calcium Oxalates from CO2 Sequestration for Storage Purposes: An In-Situ HT-XRPD and TGA Combined Study

Experiment 8 - Precipitation of CaC2O4*H2O from the Salt Mixture Unknown name Moon Stardust Limiting - Studocu
1 Chapter 3 The principle of TGA and its applications: TGA is thermogravimetric analysis. It is one of the thermal method of an

A 0.60 g sample consisting of only CaC2O4 and MgC2O4 is heated at 500 ^∘ C , converting the two salts of CaCO3 and MgCO3 . The sample then, weighs 0.465 g .
1 Chapter 3 The principle of TGA and its applications: TGA is thermogravimetric analysis. It is one of the thermal method of an