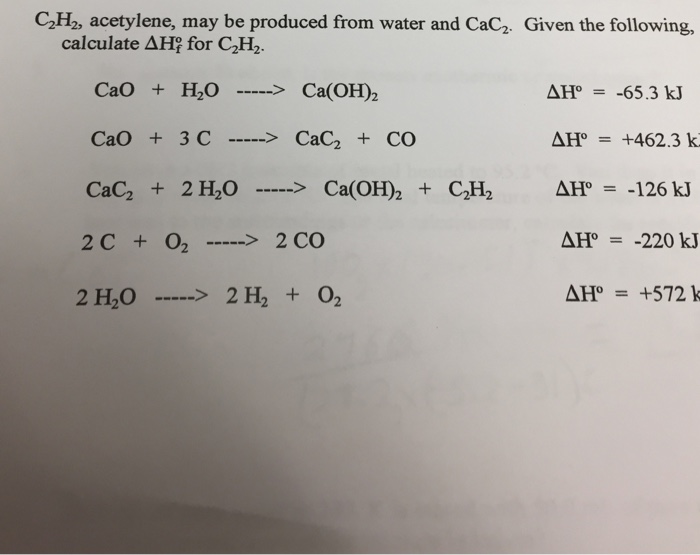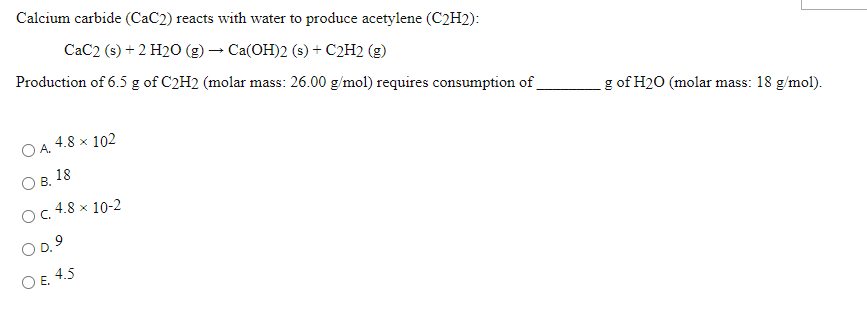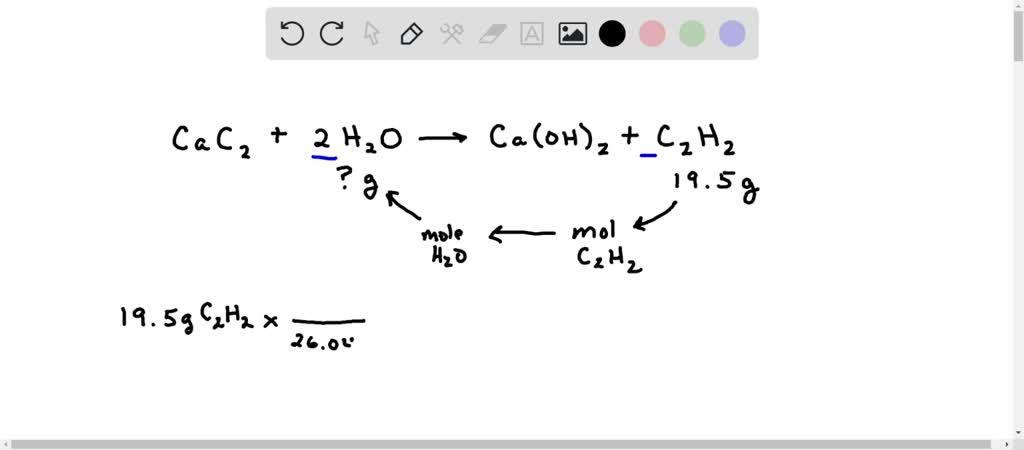
SOLVED: Calcium carbide (CaC2) reacts with water to form acetylene (C2H2): CaC2 (s) + 2 H2O (g) → Ca(OH)2 (s) + C2H2 (g) How many grams of water are required to produce

Towards C1 chemistry: methanol vinylation by CaC2 in water in the presence of potassium or sodium carbonates - Parshina - 2019 - Journal of Chemical Technology & Biotechnology - Wiley Online Library

Chemical Equations And balancing equations. Chemical Equation CH 4 + O 2 CO 2 + H 2 O Reactantsproducts Means to produce. - ppt download
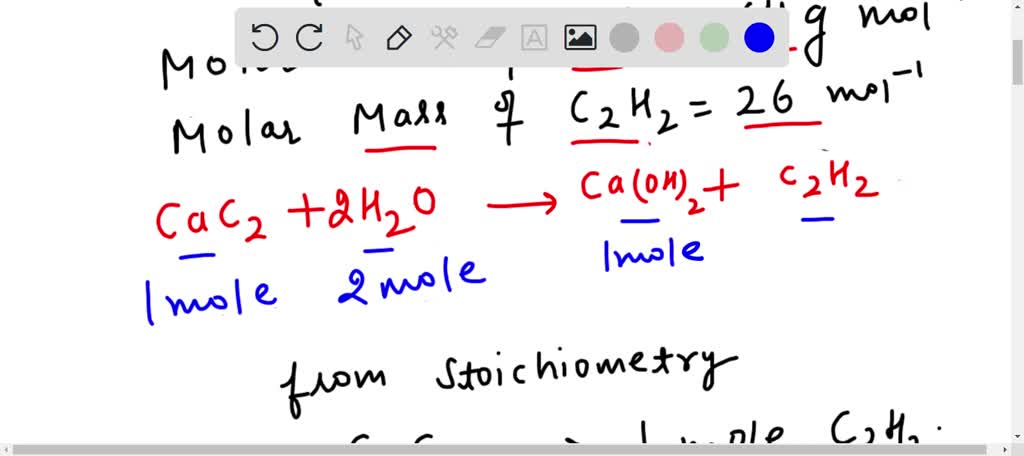
SOLVED: When 50g calcium carbide CaC2 reacts with water, what will be the volume of the product c2h2?

How to Balance CaC2+H2O=Ca(OH)2+C2H2|Chemical equation CaC2+H2O =Ca(OH)2+C2H2|CaC2+H2O=Ca(OH)2+C2H2 - YouTube

From the following reactions at 298 K .(A) CaC2(s) + 2H2O(l) → Ca(OH)2(s) + C2H2 (g); Δ H^∘ = - 127.9 kJ mol^-1 (B) Ca(s) + 12 O2(g) → CaO(s) ; Δ
CaC2 reacts with H2O and gives X. X reacts with Cu2Cl2 in the presence of NH4OH and give Y. What is Y, and how? - Quora
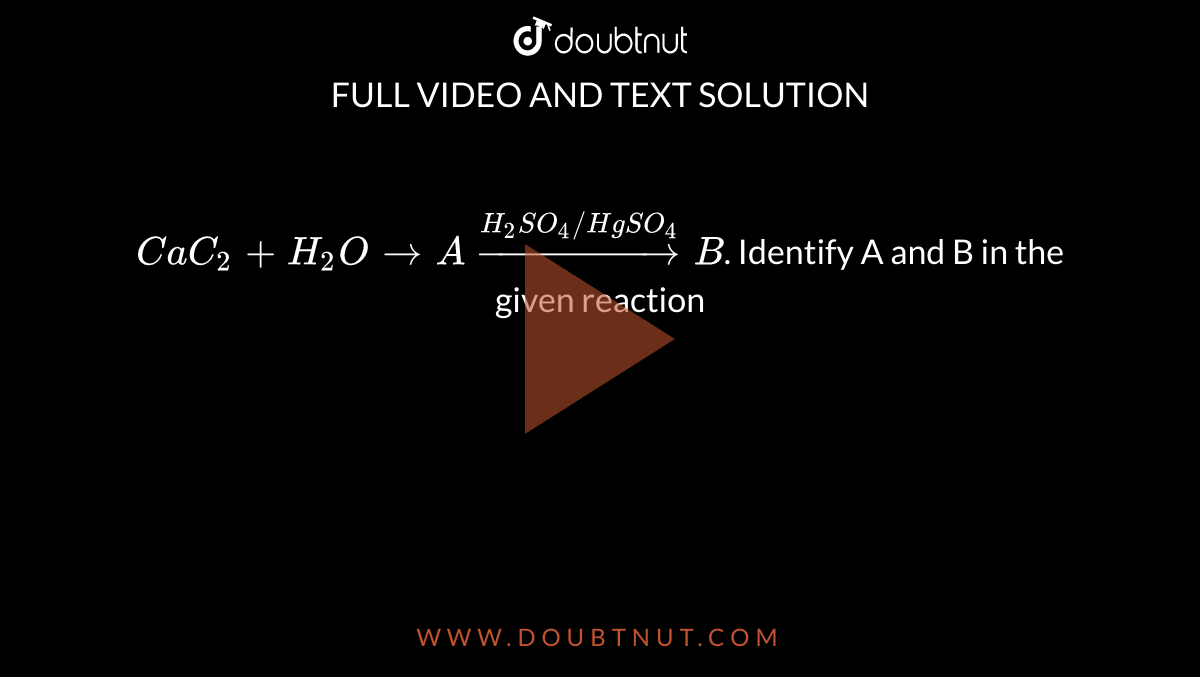
Complete the following reaction and name the products A, B and C. Cac2 + ( H2O) → A + (hot Cu tube) → B + ((conc. H2SO4 + HNO3)/(323 - 333K)) → C - Sarthaks eConnect | Largest Online Education Community